Soil pH is a critical basic tenet for successful crop production, so why do soil samples suggest so many fields are outside the target pH range? And are zero till systems more at risk?
Regenerative farmers or those not using tillage to establish crops in the rotation could be at risk of creating a low pH environment in the top 5-10cm of soils, which could impact nutrient availability, soil health, and greenhouse gas emissions.
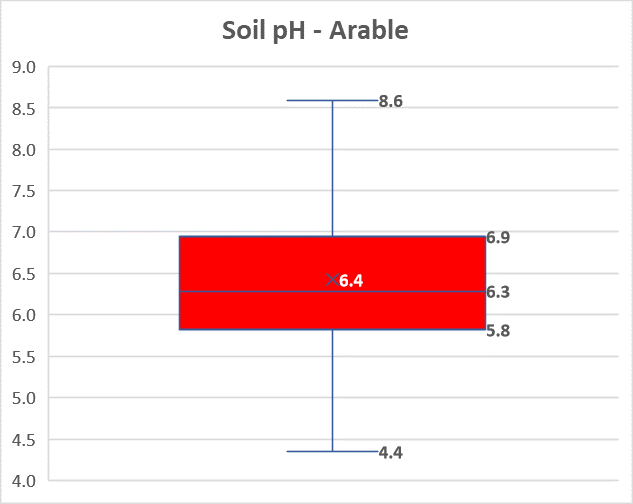
Over 50% of arable soils are below the target pH 6.7 recommended in AHDB’s Nutrient Management Guide (RB209), according to results from over 50,000 soil samples analysed by NRM laboratories between June 2022 and May 2023.
“Nearly 25% of the samples were pH 5.8 or lower, which is extremely low for most arable crops,” NRM soil and crop nutrition agronomist Sajjad Awan says. “While only around a fifth were in the more acceptable target pH range of 6.5 to 7.0.”
That probably accurately reflects industry studies suggesting arable land is only limed on average once every 12 years, way beyond the traditional recommendation of once every five years.
Longer-term data from the NRM study suggests that the pH of arable soils dropped sharply in 2022/23 compared with previous years.

“There could be multiple factors behind the drop in pH from arable soils this year,” Sajjad stresses. “It might be due to weather, an increase in samples from lower pH soils or from specific management systems. There are so many variables and the information provided with each sample is not sufficient to allow us to draw firm conclusions.”
That includes whether there is any interaction with cultivation practice, although soil experts agree that there is a higher risk of acidification, at least at the soil surface, on farms where zero tillage is used.
“If you don’t disturb the soil, you’re effectively layering or stratifying the soil,” explains Neil Fuller, technical director of the Atlas Sustainable Soils Programme.
The breakdown of organic material produces weak organic acids, so growing a lot of cover crops, incorporating straw, or using other organic amendments will tend to lower the soil pH, he says.
“So you may end up with a layer on the soil surface which is a little bit sharper in pH than the base soil. If you don’t cultivate that layer, it will potentially progressively develop through the soil.”
While some regenerative farmers are attempting to reduce the amount of synthetic nitrogen in their system, nitrogen fertilisation or fixation by legumes can be another contributory factor to lowering soil pH, notes agronomist Steve Townsend from soil management specialists Soil First Farming.

“If you look at the ammonium part of ammonium nitrate, when that gets taken into the plant, the plant at root level snips off two hydrogens and takes in amide, which is NH2 rather than NH4.
“Those two hydrogen atoms go back into the soil and cause the soil to become more acidic as more of the cations are filled up with hydrogen rather than calcium, magnesium or potassium.”
Similarly, the denitrification of nitrate can cause acidity, while high rainfall, such as we’ve had this winter and spring, can leach calcium and magnesium through the soil, leaving a higher concentration of hydrogen ions attached to clay particles.
Optimum soil pH is crucial for nutrient uptake. Most arable crops like slightly acidic conditions, hence the target of pH 6.7, as that is when most nutrients are available for plants to absorb.
As soon as soil pH is below the target, fertiliser efficiency drops, as it is locked up or lost, with plants not having access to it. For example, at pH 6, nitrogen efficiency drops to 89% compared with pH 7, while phosphorus is even more affected at just 52% efficiency. At that pH, nearly 20% of fertiliser is wasted, a figure which increases to 32% at pH 5.5, according to AHDB figures.
Micronutrients can similarly be affected, Neil says. “At high pH or low pH they change form or function. For example, manganese at a low pH is a four positive cation, while as it moves towards pH 6.5, it becomes two positive, which is what the plants need it as.”
It’s good farming practice to keep your pH where it needs to be, Steve stresses.
It’s not just about pH, he adds, but also about calcium. “If you want soil biology to grow and flourish, you need enough available calcium. We use calcium for our bones, soil biology uses it for its skins.
“It’s also important as a nutrient for crops for rooting, to help nutrition be picked up and for soil structure. Of the cations in the soil, calcium is the biggest and is the only one that improves soil structure through flocculation.
“It has two positive charges and holds onto a negative bit of clay on either side, which it holds there. We see that as tilth and crumb and improved structure.”
Choosing liming products carefully is vital, he warns. “Look at reactivity rather than neutralising value.”
Reactivity quantifies the effectiveness and speed of reaction of a liming material.
“Reactivity equals grind size; how finely ground the lime is you’re buying.”
Research by the University of North Carolina shows that pH continues to drop following lime coarser than 0.84mm, while between 0.3mm and 0.84mm, it takes 15-18 months post-application for pH to peak. In contrast, material between 0.15 and 0.18mm will react within six months and hold pH at that level, and even finer lime will increase pH immediately, peaking at eight months before beginning a slow decline.
British Sugar’s LimeX meets that spec, says the firm’s LimeX business manager, Glenn Carlisle. LimeX is a co-product of lime being used within the sugar purification process to remove impurities from sugar juice.

“You end up with a very fine particle-size lime product, making it very reactive. In Ground Lime quality standard tests, it scores 100% on the reactivity test, meaning that 100% of the product you put on land will do the job of pH amendment.”
As a minimum, 85% of the LimeX product will pass through a 0.15mm sieve, with 97% passing through a 3.35mm sieve. At that spec, Glenn says in the right conditions pH should rise within 4-6 weeks, whereas other products can take from six to 12 months before you see a rise.
“The additional benefit of the fine particle size is that the calcium is more available, and you can increase the available calcium concentration in the soil. That has additional benefits in terms of flocculation and soil conditioning, which benefits aeration, drainage, microbial activity and provides a calcium nutrition source for the plant, which helps natural disease control,” Glenn concludes.