Written by Joel Williams
In the last article we introduced the various habitats that exist on and within plant tissues where a range of microbes coexist with plants and provide many benefits to growth and development. Despite the majority of microbiota living around plant root systems, there are also a range of microbes that also uniquely associate with plant shoots, leaves, flowers or seeds; and we are only just beginning to understand their importance. In this article we will take a closer look specifically at the seed microbiome and explore some of the factors that shape this biome and how this can be of benefit toward a more sustainable agriculture.
Like many other examples in agriculture where we have tended to focus on the negative, the prevalence of pathogens on seeds has been extensively studied and has dominated much of the thinking regarding seed microbiota. However, the occurrence and role of other beneficial microorganisms – which constitute a majority of the seed associated organisms – are relatively unknown. Seeds generally present similar proportions of bacterial and fungal diversity, which contrasts with other aboveground plant compartments that are for the most part, highly dominated by bacterial diversity. Microbial communities associated with the seed coat are usually more diverse than those associated within the seed – only a smaller number of specialist microbial species have the ability to pass through the external barriers of plant tissues and colonise tissues within; most others can only associate with the external surfaces.
In the same way that there are unique and distinct microbes that associate with different plant parts, there are also specific microbes that associate exclusively to distinct micro-habitats of the seed itself. There are three seed compartments where microbes associate – the embryo, the endosperm and the seed coat. Seed-associated microorganisms can be acquired either ‘horizontally’ from various and local environments (e.g. air, water, insects, seed processing) or ‘vertically’ passed down from the mother plant, and hence, transmitted across multiple generations. Overall, microbes associated with the embryo and endosperm (internally) are more likely to be transmitted vertically than those associated with the seed coat, these being mostly transmitted horizontally.
Three main transmission pathways have been documented:
1. The internal pathway – whereby microorganisms colonise developing seeds via the xylem or nonvascular tissue of the mother plant.
2. The floral pathway – whereby microorganisms colonise developing seeds via the xylem or nonvascular tissue of the mother plant.
3. The external pathway – that represents microbial colonisation of developing seeds through the stigma.
Of course, the development and application of the majority of seed treatment technologies have focussed primarily on the external pathway. Some of these inoculants are designed to remain on the outside and colonise the roots as they develop while some are destined to become endophytes and enter the plant tissues (such as rhizobia or some mycorrhiza for example). The exact mechanisms which determine the final structure and composition of the seed microbiome are still being elucidated but factors that influence this include a range of environmental conditions, soil type and perhaps most importantly, the host plant itself plays a major role in shaping its seed microbiome.
It is now understood that each and every plant species recruits and structures a microbiome unique to that species (referred to as its core microbiome), and even going beyond this, different varieties also shape their own ‘varietal specific’ microbiomes. These kinds of insights are opening some fascinating doors to understanding the species specific nature of plant-microbe interactions, which in the future will no doubt help design efficient production systems whereby plant varieties and microbial strains are highly aligned and optimised for various outcomes (plant health, pest resistance, nutrient use efficiencies etc). Although I fully support the use of highly diverse, broad spectrum and DIY inoculants like compost extracts, there are many examples whereby successful suppression of a pathogen (for example) is dependent on a specific antagonistic mechanism from one particular microbial species (or even strain); so illuminating some of these highly specific crop-microbe interactions at the molecular level will be a fruitful endeavour in years to come.
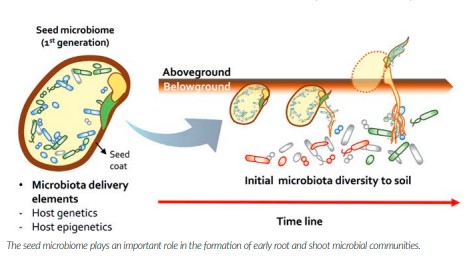
In the meantime, it is clear that the seed microbiome is of utmost importance to plant development – affecting growth, drought resistance, disease resistance and even flowering times. We know the seed microbiome becomes active immediately after sowing as the germination process begins. These microbes associated with the seed are the early risers so to speak and consequently play a key role – somewhat as gatekeepers – in safeguarding the seed and communicating to the rest of the soil biome and shaping which organisms from the soil can or can’t subsequently colonise the seed and the emerging roots and shoots.
This early structuring of the microbial community that subsequently colonises the plant can have major and long-lasting implications on how the root and shoot microbiome matures through the rest of the plant developmental stages. There are major knowledge gaps on the impact of fungicidal seed dressings on the nontarget organisms of the seed microbiome. We can safely assume that at least some beneficials will be compromised but whether the use of such inputs may be leading to negative consequences – such as greater disease susceptibility – in later crop stages remains to be studied. Even less understood is whether fungicidal dressings may be impacting the composition of the seed microbiome that is subsequently inherited from the mother plant to the next generation and hence inducing transgenerational changes in the seed microbiome over time.
Practically speaking, there are 3 take homes we can draw from these insights.
1. Eliminate the use of fungicidal seed dressings – if this idea is too daunting for you, start small. Choose half a field or a few tramlines and start the process on a small scale. Observe as you go and scale up in stages that are comfortable within your attitudes to risk.
2. Substitute dressings with bioinputs – rather than just cut out dressings, it really is preferrable to substitute the chemical with other biostimulants or bioinoculants. These could also be applied to the seed or injected into the furrow where possible. Input substitutions might include humic acid, fish hydrolysate or molasses along with some kind of microbial inoculant such as compost extracts or commercial products.
3. Save your own seed – considering that part of the seed microbiome is inherited from the local environment (mostly the soil), saving seed from plants that were grown in your soil is potentially optimising the microbiota that associate with your seeds to your specific soil type, growing conditions and management practices. There is still much to learn regarding these potential transgenerational effects but early indications suggest this is worth pursuing.
References
1. The variable influences of soil and seed-associated bacterial communities on the assembly of seedling microbiomes. (2021). doi:10.1038/s41396-021- 00967-1.
2. Seed microbiota revealed by a large-scale metaanalysis including 50 plant species. (2021). doi:10.1101/2021.06.08.447541.
3. Plant Communication With Associated Microbiota in the Spermosphere, Rhizosphere and Phyllosphere. (2017). doi: 10.1016/ bs.abr.2016.10.007
4. Inheritance of seed and rhizosphere microbial communities through plant–soil feedback and soil memory. (2019). doi: 10.1111/1758- 2229.12760
5. Revisiting Plant–Microbe Interactions and Microbial Consortia Application for Enhancing Sustainable Agriculture: A Review. (2020). doi: 10.3389/fmicb.2020.560406