By James Warne, Soil First Farming
I recently attended the appropriately entitled ‘Calcium Conference’ which served to remind me of the importance to distinguish between calcium and pH balance within our soils, and also the importance of calcium to the dairy cow. How easily we confuse the use of calcium has with the role of pH management within soils simply because we regularly test soil pH and then apply a calcium containing product to correct the pH.
So let me deal with this confusion. pH measures the concentration of hydrogen ions in the soil solution (when the pH is below 7) and adsorbed on the clay colloids (no mention of calcium there!) As the concentration of hydrogen ions in the soil solution rises exponentially the pH falls. For example as the pH drops from pH 6 – pH 5 the concentration of Hydrogen ions increases tenfold and if it drops from pH 6 –pH 4 the concentration of hydrogen ions increases by 100. To neutralise the hydrogen ions we most usually apply lime, or calcium carbonate in its various forms. The neutralising bit here is the carbonate, NOT the calcium. The carbonate disassociates from the calcium in the soil and reacts with the hydrogen to leave carbon dioxide and water while the calcium ion replaces the hydrogen in the soil solution and on the clay colloids. To get scientific for a moment the reaction is
CaCO3 + H2 -> Ca2+ + CO2 + H20
Calcium carbonate in the soil reacts with the hydrogen ions (the acidity) to give calcium ions, carbon dioxide and water. So if the calcium does not neutralise the acidity why should I be worried about my soil calcium levels? Calcium is the sixth most abundant element in plants, and animals for that matter.
It is also the third most abundant metal in the earth’s crust. Historically it has always been assumed that there is generally enough calcium in the soil to supply the crop requirements if the soil is limed to the correct pH. However we also loose calcium from the soil in large amounts every year by a variety of processes. Crop removal via mechanical or animal means removes varying amounts of calcium but these losses is quite small compared to losses caused by inorganic fertilisers and natural leaching.
Work done at Rothamsted has shown that considerable amounts of calcium are lost by leaching, and the amounts lost by leaching double with every unit increase in pH, see the table below.
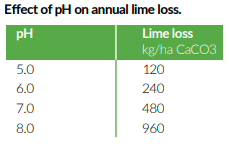
Similarly calcium can be lost by the use of fertilisers, but why is this? Most inorganic fertilisers are based around sulphates, nitrates or chlorides which are negatively charged anions when disassociated in the soil. They tend to combine with dominant cation calcium in the soil and leach readily in the soil solution. Again work done at Rothamsted suggests that every kg of N as urea and ammonium nitrate needs to be balanced with 2-3 kgs of CaCO3.
Further work needs to be done to establish an ideal model for calcium losses but it is probably somewhere in the region of 50 – 100kg per hectare per year depending upon soil type, drainage etc. You cannot rely on the soil pH to be an indicator of satisfactory soil calcium, the two do not correlate well enough for these days of precision agriculture. I have lost count of the number of times I have heard the following phrase. “I haven’t limed for several years, each time we check the soil pH the results show we don’t need to lime”. But when a cation exchange analysis is undertaken the soil can be very low in calcium. Other factors are keeping the pH at a satisfactory level, which are detrimental to yield and soil health.
So why is calcium so important within the soil?
Firstly as mentioned above calcium is the dominant cation within the soil and as such plays an important role in soil aggregation due to the way its ionic charge, size and hydration acts upon the clay colloids. Soil aggregation or flocculation is the process by which soil colloids and organic matter clump together to form aggregates. It is these aggregates, or crumb structure, which we call tilth. It is this aggregation of the soil that creates pore space within the soil and consequently it is the pore space which allows water and air to move through the soil. As we all seem to be preoccupied with ‘soil health’ at present I always feel that calcium is a good starting point as it drives so many of the other factors which contribute to soil health.
Calcium is essential too for the soil biology (another function of soil health) The beneficial soil biology are generally aerobic (i.e. they need oxygen to function). It is the porosity created as described above by the calcium that allows the movement of oxygen to allow the biology to flourish. Equally the biology also requires large amounts of calcium to support their bodily functions.
Calcium within the plant..
All plants require calcium, it is an essential nutrient along with nitrogen, phosphate, copper etc etc. In fact it is the sixth most important element by concentration within plant tissues. Calcium uptake maybe affected by the presence of other cations in the soil such as potassium, magnesium, ammonium etc. Similarly though excess calcium may reduce the uptake of these cations too, so it’s not simply a case of over applying in the hope that it may do some good. Excess soil calcium can cause as much trouble as deficiency.
Calcium is mainly taken up with the soil water and moves within the xylem stream. Consequently when the transpiration rate is reduced calcium intake may suffer. Parts of the plant with a low transpiration rate may also be low in calcium such as the fruits. Once used within the plant calcium is reasonably immobile, consequently crops require a continuous availability of calcium. Calcium is an important element within the cell wall structure where it forms calcium pectate which give stability and bind cell walls together.
Calcium pectate give the cells walls their structure and strength and gives the plant its standing strength and a higher resistance to fungal infections and aphid borne virus. Many fungi and bacteria secrete enzymes which impair cell wall integrity, calcium can help to reduce this effect. Calcium also plays an important part in fruit quality and starch formation amongst other functions. Being immobile deficiency symptoms show in the younger leaves and plant issues first. Typical symptoms are stunting of plants and lack of leaf expansion.
Useful forms of calcium
In agriculture terms we really only use three forms of calcium containing product, lime, chalk or gypsum. Lime can be further divided into calcitic or dolomitic lime. Calcitic lime contains calcium carbonate, as will chalk, while dolomitic contains calcium and magnesium carbonate. Gypsum contains calcium sulphate. All of these products are crushed and ground from rock and consequently the quality can be very variable depending upon the source. To enable these products to work effectively they need to be very finely ground to dust.
Otherwise the release of calcium cannot be predicted. Dust is particularly difficult to spread accurately unless it is formed into a prill. Calcium carbonate also has a low solubility compared with gypsum, therefore the calcium will be much more readily available from the gypsum compared to the lime. Another overlooked source is CAN, once a widely used fertiliser, but now consigned to horticultural crops it is a useful source of calcium plus the ammonium nutrition which is altogether better for crop health than nitrate.
Finally…
In conclusion, if you are trying to build a healthy soil and grow a healthy crop, calcium should be your first consideration, and just because your pH is correct don’t assume you have the correct level of calcium for your soil. And finally, I don’t sell calcium products but most soil tests I receive do suggest that our soils are in need of calcium.
References
Principles of Plant Nutrition 2001. Mengel
& Kirkby
Crop Nutrition & Fertiliser Use 1996.
Archer